Для цитирования:
Набила Эль Аллали Эль Хамдауи, Милена Кнежевич, Милан Кнежевич, Марио Мануэль Висенте-Барреро. Поперечное исследование и анализ контаминации и дезинфекции зубных щеток. Исследование 101 зубной щетки, использовавшихся людьми разных возрастов. Голова и шея. Российский журнал Head and neck. Russian Journal. 2020;8(2):45–51
For citation: Nabila El Allali El Hamdaoui, Milena Knezevic, Milan Knezevic, Mario Manuel Vicente-Barrero.
Cross section study and analysis of toothbrushes contamination and disinfection. Study of 101 toothbrushes emloyed from the people of different ages. Head and neck. Russian Journal. 2020;8(2):45–51 The authors are responsible for the originality of the data presented and the possibility of publishing illustrative material – tables, figures, photographs of patients.
Введение: Зубные щетки стали обязательным и необходимым атрибутом человеческой жизнедеятельности и доказали свою эффективность в устранении бактериального налета. Настоящее исследование посвящено оценке контаминации этого жизненно важного предмета, поскольку на зубной щетке могут локализоваться микроорганизмы, что делает ее возможным источником инфекции для человека. Кроме того, населению важно рекомендовать тот метод дезинфекции, который будет достаточно щадящим для щетки, поэтому в данном исследовании в качестве дезинфицирующего средства мы использовали хлоргексидин.
Цель: изучить и описать результаты, полученные при микробиологическои культивировании серии образцов из зубных щеток, а также эффективность 0,12% хлоргексидина в деконтаминации изученных зубных щеток.
Методология. Проведено поперечное исследование, в котором были изучены зубные щетки 101 человека разного возраста. В процессе исследования щетинки указанных зубных щеток собирали по отдельности и в стерильных условиях, а затем транспортировали в Микробиологическое отделение Больничного Комплекса Университета Матери и Ребенка, где после посева определяли тип загрязнения. От каждого участника был получен заполненный опросник с различными переменными, а также информированное согласие на исследование. Кроме того, 36 образцов были погружены в раствор хлоргексидина для оценки эффективности деконтаминации.
Результаты: 47,5% проанализированных зубных щеток были контаминированы обычной микробиотой рта и 27,7% – грамотрицательными бациллами. Хлоргексидин снижал бактериальную нагрузку и даже полностью дезинфицировал некоторые зубные щетки.
Выводы. В 75% образцов с зубных щеток, несмотря на то что зрительно они были в хорошем состоянии, были выделены микроорганизмы – участники обычного микробиоценоза ротовой полости, а также грамотрицательные бациллы. Все электрические зубные щетки были загрязнены привычной микробиотой рта и грамотрицательными бациллами. Было показано, что растворы хлоргексидина снижают бактериальную нагрузку на зубные щетки; их использование в качестве метода дезинфекции является простой в выполнении рекомендацией.
Ключевые слова: зубные щетки, хлоргексидин, дезинфекция
Конфликт интересов. Авторы заявляют об отсутствии конфликта интересов.
Финансирование. Работа выполнена без спонсорской поддержки
Для цитирования: Набила Эль Аллали Эль Хамдауи, Милена Кнежевич, Милан Кнежевич, Марио Мануэль Висенте-Барреро. Поперечное исследование и анализ контаминации и дезинфекции зубных щеток. Исследование 101 зубной щетки, использовавшихся людьми разных возрастов. Голова и шея. Российский журнал = Head and neck. Russian Journal. 2020;8(2):45–51
Авторы несут ответственность за оригинальность представленных данных и возможность публикации иллюстративного материала – таблиц, рисунков, фотографий пациентов.
Introduction: The toothbrush has become the obligatory and essential complement of the human being and has proven to be effective in the elimination of the bacterial plaque. The present study seeks to know the contamination of this vital tool, since the toothbrush can accommodate microorganisms and therefore become a possible source of infection for the individual. Also, it is important to recommend to the population some method of disinfection to properly preserve this element, therefore, in this investigation we will use chlorhexidine as a disinfectant.
Objective: To study and describe the results obtained from the microbiological culture of a series of toothbrush samples as well as the effectiveness of 0.12% chlorhexidine in the decontamination of said toothbrushes.
Methodology: Cross-sectional study in which the toothbrushes of 101 people of different ages were included. For this, the bristles of said toothbrushes were collected individually and under sterilized conditions, and then transported to the Microbiology service of the Maternal and Child University Hospital Complex, where the type of contamination after the crop was identified. Each subject was given a survey with different variables, as well as informed consent for the study. In addition, 36 samples were immersed in a solution of chlorhexidine to assess the effectiveness of decontamination.
Results: 47.5% of the toothbrushes analyzed were found to be contaminated by the usual microbiota of the mouth and 27.7% by gram-negative bacillus bacteria. Chlorhexidine reduced the load on the bacterial load and even managed to completely disinfect some of the toothbrushes.
Conclusions: In 75% of toothbrush samples, despite being apparently well preserved, microorganisms of the usual microbiota were isolated from the mouth and gram-negative bacilli.
- All electric toothbrushes were contaminated by habitual microbiota of the mouth and gram-negative bacilli.
- Chlorhexidine solutions have been shown to reduce the bacterial load of toothbrushes because it would be convenient to recommend their use as a disinfection method.
Key words: Toothbrushes, Chlorhexidine, Disinfection
Conflicts of interest. The authors have no conflicts of interest to declare.
Funding. There was no funding for this study.
For citation: Nabila El Allali El Hamdaoui, Milena Knezevic, Milan Knezevic, Mario Manuel Vicente-Barrero.
Cross section study and analysis of toothbrushes contamination and disinfection. Study of 101 toothbrushes emloyed from the people of different ages. Head and neck. Russian Journal. 2020;8(2):45–51
The authors are responsible for the originality of the data presented and the possibility of publishing illustrative material – tables, figures, photographs of patients.
Introduction
The oral cavity is composed of several surfaces (saliva, mucosa, teeth, periodontal groove and tongue) and each of them hosts different types of microbiota. In addition, it offers easy access to the bacteria, viruses and fungi of the environment, being one of the areas that hosts most microorganisms in the human body, managing to isolate up to 200 different species from the same oral cavity [1].
Currently, the majority of the population spends daily a few minutes of their lives for the maintenance of oral health. To do this, they use the toothbrush, a tool that serves to achieve proper oral hygiene, along with other elements such as dentifrices, dental floss, mouthwashes, etc. The toothbrush, in theory, is the main element that seeks to achieve the reduction of the microbial load, the prevention of dental diseases, such as tooth decay, and the promotion of good oral health. But is our toothbrush clean?
It is known that toothbrushes can be contaminated by different microorganisms, which not only come from the mouth, but also from the environment, from the hands, from the storage place, either in the bathroom itself or outside it, as in a case, of the dispersed aerosols released from the toilet [2, 3] and also from the direct contact of the toothbrushes of the same family that are stored together in the same container or place [4].
Numerous studies have shown that prolonged use of the toothbrush facilitates its contamination by various microorganisms such as streptococci, staphylococci, lactobacilli, gram-negative bacilli (Pseudomonas, Klebsiella, Escherichia coli) and yeasts (Candida). It has been seen that these microorganisms grow better in hot and humid conditions, such as those we can find in our bathrooms [2, 5].
The microorganisms can survive in the bristles of the toothbrushes for periods of 24 hours to 7 days [3], creating a vicious cycle of reinfection, and for this reason, causing possible oral diseases, as well as leading to producing serious systemic diseases such as infective endocarditis [6].
the population is not aware of the importance of proper toothbrush maintenance [5–7]. There is no scientific agreement on how to keep our toothbrush in optimal conditions. We simply know that we should replace the toothbrush every 3 or 4 months, or sooner if the bristles are damaged, as recommended by the American Dental Association [8].
Therefore, it is important to teach the population all available decontamination methods. Wash the brush with bactericidal solutions (alcohol, cetyl pyridinium chloride, polyvinyl, pyrrolidone and chlorhexidine among others), wash the brush in running water after use, apply ultraviolet and microwave light, and apply bactericidal agents on the bristles [9] These are some of the procedures that can be used to control the contamination of toothbrushes. Currently, some of these measures are rarely used, which is why dental brushes with antibacterial agents have been introduced to control such contamination [10].
Objectives
The objective of this study is to investigate the level of contamination of toothbrushes of a group of people with different conservation conditions. Also, we seek to analyze variables such as age, sex, storage location, the way of cleaning after use, the type of brush, the months of use and the existence of caries. Finally, we want to evaluate the effect that a disinfectant has, in this case 0.12% chlorhexidine on some samples, in order to determine how much the microorganisms found can be eliminated and thus demonstrate that it can be used as decontamination method.
Methodology
The study presented here is a descriptive transversal type, which seeks to examine the type of contamination of toothbrushes and evaluate the possibility of disinfection.
Toothbrushes were randomly collected from 101 people, who voluntarily agreed to participate in the study. Also, the informed consent of the participants was requested, after explaining all the information about the investigation (Annex).
Each person was given a following survey with specific data to be analyzed were collected:
– Age.
– Sex
– Toothbrush storage place (bathroom drawer, in the bathroom or outside the bathroom).
– Saved with case or not. – Way to clean the toothbrush after use.
– Type of toothbrush (manual or electric).
– Months of use of the toothbrush (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or more than 12 months).
– Existence of caries.
scalpels to trim several bristles from the brush head, droping the filaments directly into a sterile bottle and avoiding external contaminants. Immediately, the bottles were ordered sorted according to the patient survey number.
The jars with the bristles inside were stored in the refrigerator at 4°C for a maximum of 24 hours. Subsequently, these samples were transferred to the Microbiology Service of the Las Palmas University Hospital Complex.
Once in the laboratory, the filaments of the toothbrushes were droped in liquid medium, type “Brain Heart Infusion” (BHI) and placed in the oven at 35°C for 24 hours, to favor the growth of microorganisms. After this incubation time, liquid cultures were seeded in solid medium (blood agar, chocolate agar and McConkey agar), and said Petri dishes were incubated in the oven at 35°C. After 24 hours, the data were interpreted with the help of an optional specialist from the Las Palmas University Hospital’s Microbiology Service, and the results were classified into three groups: a) toothbrushes with negative culture; b) toothbrushes contaminated by usual oral microbiota; and c) toothbrushes contaminated by gram-negative bacilli (GNB), which are microorganisms from intestinal microbiota.
Additionally, in 36 random samples of liquid culture medium (BHI) that were obtained after incubation in the oven, 0.12% chlorhexidine of the Lacer® brand was added, in order to carry out the disinfection process. The mixture was allowed to stand for 10 minutes and then seeded in solid medium (chocolate agar). The plates were introduced in the stove and after 24 hours the results were interpreted.
All the information in this investigation was saved in a Microsoft Excel sheet and the statistical analysis was performed using the R package, version 3.3.1 (R Development Core Team, 2016). Statistical significance was established at p 0.05.
Results
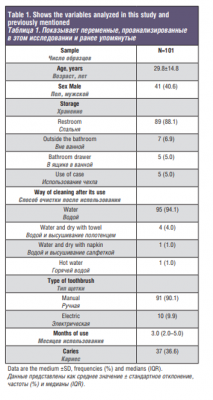
The 101 people who participated in the study had an average age of 29.8 years and a standard deviation of 14.8. A total of 60 women and 41 men participated, being 59.4% and 40.6%, respectively. Most of them kept the toothbrush in the sink (88.1%) and used a manual brush (90.1%).
Regarding the way to clean the toothbrush after use, participants referred to it in several ways, some cleaned with water and dried with a towel (4%) or napkin (1%), others put the brush in hot water (1%), and most washed the brush under running water from the tap (94.1%).
Of all the members of this study, only 5% used a case to transport the toothbrush and 36.6% had cavities. The average number of months used of toothbrushes in this study has been 3 months (2.0–5.0).
Table 1 shows the variables analyzed in this study and previously mentioned. After the culture of the samples, three groups of contamination were identified, predominantly toothbrushes with habitual microbiota of the mouth (47.5%). Next, it was observed that 27.7% of toothbrushes were contaminated by gram-negative bacilli and 24.8% were negative, since they were totally clean. As we can see, the results that have been obtained none were significant, therefore, we cannot demonstrate the relationship between the variables measured with the types of contamination found.
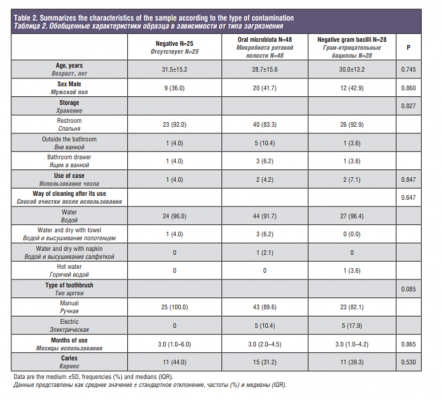
Table 2. Summarizes the characteristics of the sample according to the type of contamination.
Of the 25 toothbrushes that had no contamination, 16 (64%) belonged to women and 9 (36%) to men. In addition, it should be noted that most toothbrushes were stored in the sink (92%) and a minority, in the bathroom drawer (4%) and outside the bathroom (4%). Only one subject (4%) used the case to carry his toothbrush. In relation to the way to clean the toothbrush after use, almost all cleaned with tap water (96%), except in a case that washed with water and dried with a towel. Finally, 11 (44%) people had caries lesion in this pollution group.
In the group of toothbrushes contaminated by habitual microbiota of the mouth, 48, 28 were women and 20 men, that is, 58.3% and 41.7% respectively. Of these, 40 (83.3%) kept their toothbrush in the sink, 3 (6.2%) in the bathroom drawer and 5 (10.4%) outside the bathroom. Only two people (4.2%) used the case. In this group, 44 (91.7%) cleaned the brush with water, 3 (6.2%) dried with a towel and 1 (2.1%) with a napkin. 31.2% had tooth decay.
The rest of the sample studied, 28 in total, were contaminated by gram-negative bacilli that come from intestinal microbiota, either by the environment itself or by the way of maintenance of the brush or by the aerosols of the environment. 16 (57.1%) brushes corresponded to women and 12 (42.9%) to men. 26 people (92.9%) kept their toothbrush in the bathroom and only one kept it in the bathroom drawer (3.6%) and another outside the bathroom (3.6%). The case has been used by two people (7.1%). Once again, the majority (96.4%) cleaned the brush with water and only one with hot water (3.6%). 39.3% carried tooth decay in their mouths.
Regarding the variable type of brush, all those that were completely clean were manual brushes. Of the 10 electric brushes examined in this study, half were contaminated by usual microbiota and the other half by gram-negative bacilli. This variable almost turned out to be significant (p = 0.085).
The average age and the months of use that were calculated for each contamination group coincide practically with that calculated for the total.
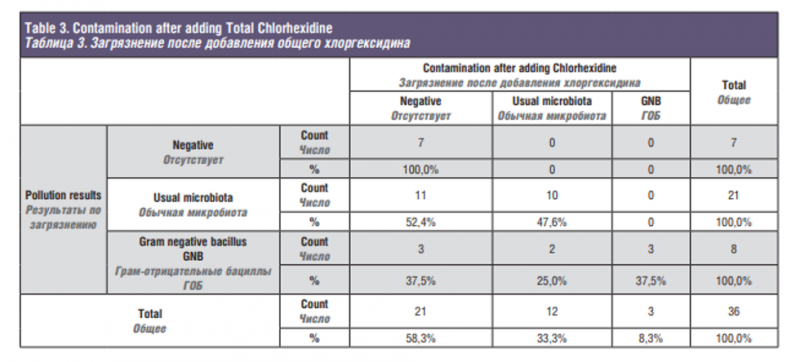
Finally, Table 3 shows how chlorhexidine works on 36 brushes with different types of contamination. It should be noted that chlorhexidine has managed to reduce the microbial load, since 8 samples containing gram negative bacilli, two became of contamination with usual microbiota and three were completely decontaminated. Of the samples with usual microbiota, 21 in total, 11 were completely decontaminated. Evidently on the brushes with negative contamination the chlorhexidine has not caused any effect.
Discussion
In our research we have not studied specific microorganisms; we have limited ourselves to classifying them according to the type of contamination. 24.8% of the toothbrushes analyzed were free of contamination, unlike some studies that have seen that practically the entire sample wasAlmost half of the samples, evaluated, indicated positive for bacteria typical of the usual flora of the mouth, coinciding with the studies by Svanberg [12] and Raiyani et al. [4] which state that Streptococcus mutans, a bacterium that is part of the usual oral microbiota, was the most found in toothbrushes. In a different way, Almutairi et al. [6] demonstrated the presence of Staphylococci in large quantities. Although they belong to the oral microbiota, contaminated [6, 9, 11].
Staphylococcus species deserve more attention because they are capable of causing many oral infectious diseases [13–15].
Gram negative bacteria (GNB) have also been obtained in our sample, in accordance with the study by Contreras et al [10] who report them being opportunistic pathogens and capable of causing infections. According to the studies of Raiyani et al. [4] and Karibasappa et al. [16] gram-negative bacteria such as Pseudomonas, cause suppurative otitis, eye infection, urinary infection, burn infection, etc; Klebsiella causes pyogenic infection, pneumonia, septicemia, diarrhea, etc; and Escherichia сoli produces septicemia, diarrhea and urinary tract infection.
Several studies determine that the bacterial contamination of toothbrushes is generated by storing the brush in bathrooms and in humid environments, places most used, by the people in this study, for their toothbrushes. They also speculate that there is a greater risk of contamination if stored less than 1 meter away from the toilet, due to aerosols dispersed by the discharge of the tank [2, 6, 10, 17–19]. Díaz-Caballero et al. [20] state that toothbrushes are susceptible to contamination when they are located at a distance of 108 cm from the toilet and the maximum splash reaches 145 cm, thus being within the area of splash. Karibasappa et al. [16] concludes in his work that toothbrushes should not be stored in bathrooms.
Another risk factor that favors the contamination of toothbrushes and that the population does not take into account, is the proximity available with other toothbrushes that are stored together or share the same brush holder, as commonly happens in families [4, 17].
Some of the participants in this analysis exceeded the recommended use of the toothbrush. However, there was no significant relationship between the presence of contamination with the months of use. Despite this result, other authors argue that the longer it is used, the more likely it is that the toothbrush harbors microbes [17].
Another risk factor that favors contamination of toothbrushes and that the population does not take into account, is the available proximity with other toothbrushes that are kept together or share the same toothbrush holder, as is commonly the same families [4, 17].
Some of the participants in this analysis exceeded the recommended use time of the toothbrush. However, there was no significant relationship between the presence of contamination with the months of use. Despite this result, other authors argue that the longer the time of use, the more likely it is that the toothbrush will harbor microbes (17).
Most people wash the toothbrush with tap water as Marielsa Gil et al [17] and Arias Ayala et al. [21], point out, sharing this result with ours, as 95% of users clean the brush under a jet of water. As stated by Naik et al. [5] water rinsing and air drying is an incomplete procedure for toothbrush hygiene.
s an incomplete procedure for toothbrush hygiene. In this work, of the 5 people who kept the brush in a case-box after use, two were contaminated by usual mouth microbiota and the other two by GNB, what support results of others [22, 23], where is stated that the use of a case-box promotes the proliferation of microorganisms.
The electric toothbrushes examined in this study were contaminated by usual mouth microbiota and GNB. This means that they may have a tendency to contaminate easily or users of these brushes kept them longer without changing them. In the scientific literature there is no evidence on bacterial contamination of electric brushes, thus, for this reason, research is recommended on this issue. Merely, there is evidence where electric toothbrushes are compared with manuals in terms of their dexterity and function, but not in contamination.
We have not seen a statistical correlation between the presence of cavities, with bacteria present in toothbrushes. However, in our study has slightly predominated, cavities in the usual microbiota contamination group of the mouth and this may be, because Streptococcus mutans is the main microorganism involved in the cause of tooth decay in beings is also involved in the pathogenesis of certain cardiovascular diseases [5].
Although the population is not aware of the possible contamination of the toothbrush or the consequences that can occur, the scientific society studies in several ways to prevent such contamination. So far they seek to implement cheap, easy and available methods in order to be accepted by the community.
n this study we value using a chlorhexidine collusion of 0.12% of the Lacer brand® for 10 minutes for several reasons, among them it stands out that it is easy to achieve, is not expensive and also does not take long to carry out the process. After use, a decrease in sample contamination was observed. The same conclude Nelson Filho et al. [24] in their research, but soaking the toothbrush for 20 hours. Chlorhexidine is a chemical antiseptic with bacteriostatic and bactericidal properties for both gram-positive and gram-negative bacteria [5].
Susheela and Radha (2) used three types of oral and body desoffecntantes available on the market, Colgate Plax®, Listerine® and Dettol®, and concluded that, to avoid oral and general diseases, it is esential to perform decontamination of toothbrush, especially in immunocompromised people. To do this, they set to immerse the toothbrush for at least five minutes in disinfectant for sterilization.
A study by Abishek M. et al. [13] after comparing chlorhexidine with Listerine® where both of them being proved effective for disinfecting contaminated toothbrushes, pointed out that chlorhexidine is a good choice for disinfection because it is a nontoxic and easy-to-use product find like mouthwash.
Take et al. [3] used as disinfection methods, 0.2% chlorhexidine gluconate solution, ultraviolet irradiation and normal saline solution achieving with all a large reduction in dental brush contamination. They specified that ultraviolet irradiation turned out to be faster and more effective, but was expensive so it is not cost-effective to use, although it is not ruled out as a disinfection method in the future.
There is also evidence that oxygen peroxide or commonly known as oxygenated water functions as a disinfectant as it manages to control and decrease the bacterial load of toothbrushes after use.
It is common to think that covering the toothbrush with plastic caps serves to protect them from contamination, the plug can help retain moisture and promote the growth of bacteria like Pseudomonas aeruginosa [13].
- [25] significantly reduces contamination of toothbrushes, instead Nuvvula et al. [7], he says that toothpaste does not eliminate all microorganisms, but limits the microbial load on toothbrushes and therefore helps to inhibit periodontal pathogens and control the risk of bacterial infection. Therefore, the population should not settle for the exclusive use of toothpaste for pollution control, but should also supplement with sterilization techniques such as those mentioned above.
The possible limitations of this research could have been as follows: the study of a fairly heterogeneous sample, hence not having obtained significant values or statistical correlations; the number of samples studied; the use of chlorhexidine for 10 minutes, as the bristles could have been maintained longer and perhaps the negativization of the samples studied could have been achieved; memory bias, as several study participants first stated that they did not accurately remember the months of toothbrush use and therefore pointed to an approximate figure; and finally, the information obtained in the surveys when asked about the existence of cavities, because there will probably be people who could have answered badly and sbiased the data involuntarily, because they are basically not diagnosed with cavities for no keep track of the dentist and therefore they think they are exempt from it.
Conclusions
— In 75% of toothbrush samples, despite being apparently well preserved, microorganisms of usual microbiota were isolated from the mouth and gram negative bacilli.
— All electric toothbrushes were contaminated by usual mouth microbiota and gram-negative bacilli.
— Solutions with chlorhexidine have been shown to decrease the bacterial load of toothbrushes by it would be advisable to recommend their use as a disinfection method.
REFERENCES
- Cruz Quintana S.M., Díaz Sjostrom P., Arias Socarrás D., Mazón Baldeón G.M. Microbiota de los ecosistemas de la cavidad bucal. Rev Cuba Estomatol. marzo de 2017;54(1):84–99.
- Susheela P., Radha R. Studies on the mi- crobiological contamination of toothbrushes and importance of decontamination using di- sinfectants. World journal of pharmaceutical and medical research. 2016; 2:201–7.
- Tomar P., Ganavadiya R., Hongal S., Jain M., Rana K., Saxena V. Evaluating sanitization of toothbrushes using ultra violet rays and 0.2% chlorhexidine solution: A comparative clinical study. J. Basic. Clin. Pharm. 2015;6(1):12.
- Raiyani C., Arora R., Bhayya D., Dogra S., Katageri A., Singh V. Assessment of microbial contamination on twice a day used toothbrush head after 1-month and 3 months: An in vitro study. J. Nat. Sci. Biol. Med. 2015;6(3):44.
- Naik R., Telagi N., Anil B., Spoorthi B. Contaminated tooth brushes-potential threat to oral and general health. J. Fam. Med. Prim. Care. 2015;4(3):444.
- Taghreed Almutairi, Ameera Aldossary, Alhanouf Alshammari, Suaad Alwakeel, Investigations into the Microbial Contamination of Toothbrushes Isolated from Riyadh, Saudi Arabia. Adv. Environ. Biol. 2014;8(7), 2231–5.
- Nuvvula S., Mohapatra A., Nirmala S.V.S.G., Konidala U. Efficacy of various disinfectants on microbially contaminated toothbrushes due to brushing. Contemp. Clin. Dent. 2011;2(4):302.
- Cepillos de dientes – Temas A-Z — American Dental Association [Internet]. [citado 16 de mayo de 2019]. Disponible en: https://www.mouthhealthy.org/ es-MX/az-topics/t/toothbrushes.
- Jaramillo A., Aragón N., García L.M. Identificación de bacterias periodontopáticas en cepillos dentales con y sin agente antibacterial. Rev. CES Odont. 2015;28(1): 1–27.
- Contreras A., Arce R.M., Botero J.E., Jaramillo A. Contaminación bacteriana de cepillos dentales en niños y sus padres: una cuestión de educación. Rev. Estomatol. 2002;10(2):4–12.
- Sogi S.H.P., Subbareddy V.V., Kiran SND. Contamination of toothbrush at different time intervals and effectiveness of various disinfecting solutions in reducing the contamination of toothbrush. J Indian Soc Pedod Prev Dent. 2002;20(3):81–5.
- Svanberg M. Contamination of toothpaste and toothbrush by Streptococcus mutans. Eur. J. Oral. Sci. 1978;86(5):412–4.
- Mehta A., Sequeira P.S., Bhat G. Bacterial contamination and decontamination of toothbrushes after use. N.Y. State Dent. J. 2007;73(3):20–2.
- Pai V. Effect of a single-use toothbrush on plaque microflora. Indian J. Dent. Res. Off Publ. Indian Soc. Dent. Res. 2009;20(4):404–6.
- Mustafa E.A., Alnaimi R.J., Al-Talib R.A. The Microbial Contamination of Toothbrushes and Their Disinfection by Antimicrobial Solutions. Al-Rafidain Dent. J. 2008;(8):144–50.
- Karibasappa G., Nagesh L., Sujatha B. Assessment of microbial contamination of toothbrush head: An in vitro study. Indian J. Dent. Res. 2011;22(1):2.
- Gil M., Oviedo D., Rico O., Perozo E., Castrillo S., Gomez G. Enterobacterias en cerdas de cepillos dentales y exudados faríngeos de estudiantes de la Escuela de bioanálisis de la Universidad de Carabobo doi: 10.13140/ RG.2.1.5119.4403.
- Mandujano Trujillo Y. Grado de contaminación microbiana de los cepillos dentales guardados en el baño y el dormitorio de los estudiantes de odontología de la Universidad de Huanuco. Univ Huánuco [Internet]. 2018 [citado 25 de mayo de 2019]; Disponible en: http://localhost:8080/xmlui/handle/123456789/1153.
- Contreras A., Arce R., Botero J.E., Jaramillo A., Betancourt M. Toothbrush contamination in family members. Rev. Clín. Periodon. Implant. Rehabil. Oral. 2010;3(1):24–6.
- Díaz-Caballero A.J., León Barrios E.E., Montoya Vega M.E., et al. Evaluación del área de salpicadura máxima de la descarga de los inodoros, y su relación con la ubicación de los cepillos dentales en cuartos de baño en barrios de Cartagena, Colombia. Univ/ Odontol. 2002;22(47):31–6.
- Arias Ayala L.T., Hernández Suárez V.M., Aránzazu Moya G.C., Martínez López C.A. Hábitos de higiene y mantenimeinto de cepillo dental antes y después de la aplicación de un material educativo. UstaSalud. 2009;8(1):37.
- Viteri-Moya J.A. Microbial contamination in toothbrushes with and without protection of a case. Polo del Conocimiento. 2017;2(8):17.
- Medina-Patruno C., Bolaños-Rivero M., Martín-Sánchez A.M., Saavedra-Santana P., Vicente-Barrero M. ¿Cuál es el nivel de contaminación del cepillo de dientes almacenado en diferentes entornos sanitarios? Av Odontoestomatol. 2019; 35(2):69–72.
- Nelson Filho P., Macari S., Faria G., Assed S., Ito I.Y. Microbial contamination of toothbrushes and their decontamination. Pediatr. Dent. 2000;22(5):381–4.
- Efstratiou M., Papaioannou W., Nakou M., Ktenas E., Vrotsos I.A., Panis V. Contamination of a toothbrush with antibacterial properties by oral microorganisms. J. Dent. 2007;35(4):331–7.
Поступила 04.03.20 Принята в печать 25.03.20 Received 04.03.20 Accepted 25.03.20
Author information: Nabila El Allali El Hamdaoui – Medical School Las Palmas de G.C., Spain Milena Knezevic – Medical School Las Palmas de G.C., Spain Milan Knezevic – President ICMFS (International College for Maxillofacial Surgery) Mario Manuel Vicente-Barrero – Professor, Medical School Las Palmas de G.C., Spain
Информация об авторах: Набила Эль Аллали Эль Хамдауи – Медицинская школа Лас-Пальмасде-Гран-Канария, Испания Милена Кнежевич – Медицинская школа Лас-Пальмас-де-Гран-Канария, Испания Милан Кнежевич – президент ICMFS (Международный колледж челюстнолицевой хирургии) Марио Мануэль Висенте-Барреро – профессор, Медицинская школа Лас-Пальмас-де-Гран-Канария, Испания